United Nations international year of the periodic table of chemical elements: June - silicon
, 30 June 2019
Into June and we have selected silicon as our element of the month. This element might not be instantly recognisable as of significance to Wales, but it does have an interesting history.
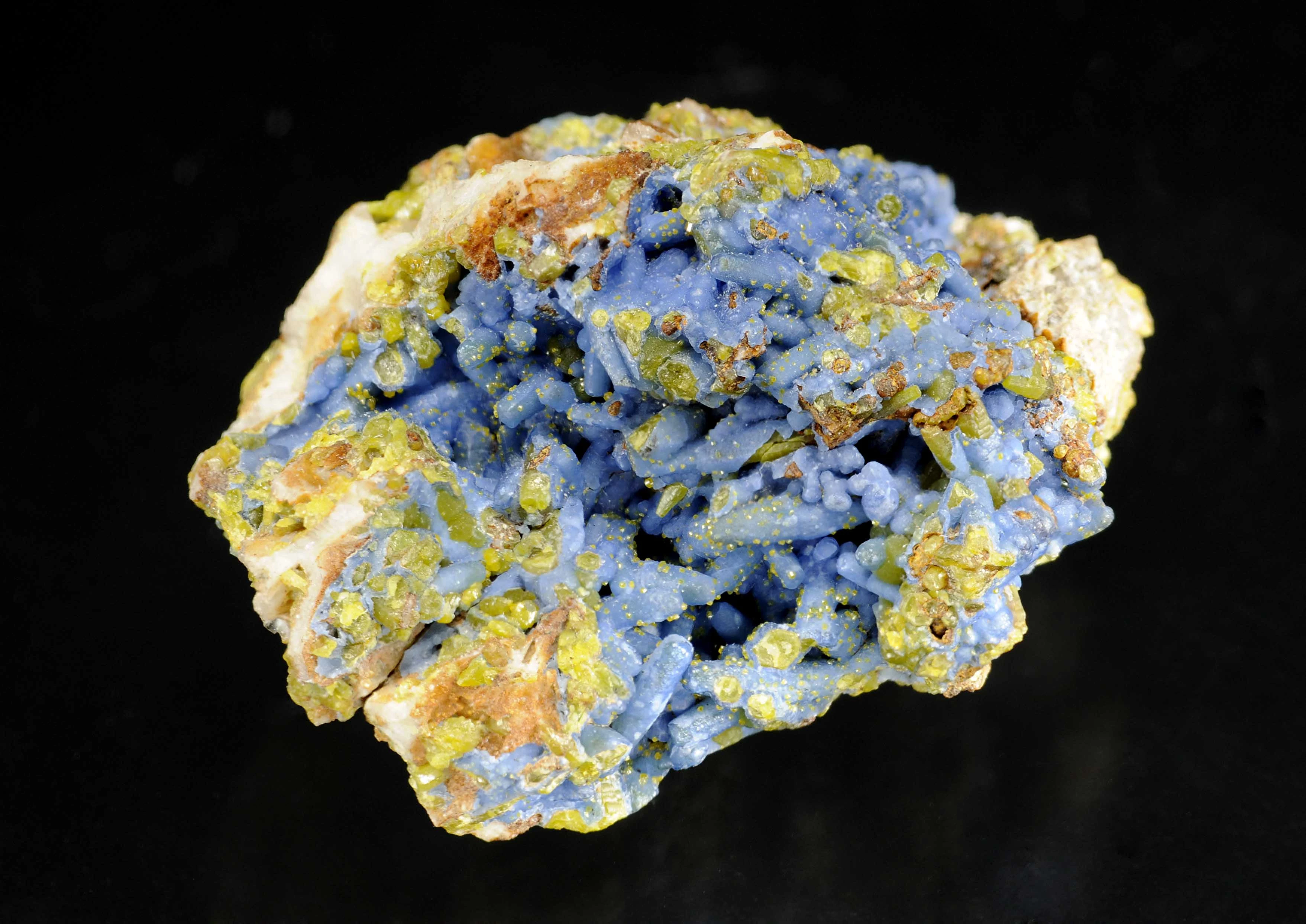
Silicon (chemical symbol – Si), atomic number 14, is a hard but brittle crystalline solid, with a blue-grey metallic lustre. Silicon is the second most abundant element (about 28% by mass) in the Earth’s crust after oxygen with which it has a strong affinity. Consequently, it took until 1823 for a scientist - Jöns Jakob Berzelius – to prepare it in pure form.
In Wales, silicon is present virtually everywhere in one form or another: from quartz (silicon dioxide, SiO2) in sedimentary siltstones, sandstones and conglomerates; complex silicates in igneous and metamorphic rocks; to sediments in soils.
Silica (silicon dioxide, or quartz) was mined extensively in the Pontneddfechan area, in South Wales, from the late 18th century until 1964 for the manufacturing of firebricks for kilns and furnaces. It occurs as a very pure material highly concentrated in quartzite within a geological unit known as the Basal Grit. Weathering and erosion of the quartzite has produced deposits of silica sand and this was extensively quarried for the production of refractory fire bricks for the smelting industries.
In North Wales, a little-known trade in rock crystal – a colourless, glassy variety of quartz crystal – took place in Snowdonia during the 18th and 19th centuries centred upon the village of Beddgelert. T. H. Parry-Williams refers to this in one of his writings. Miners and mountain guides searched for veins of quartz in the mountains and collected crystals to sell to tourists as curios and some were possibly used to make crystal chandeliers. Later, crystals were occasionally discovered in the vast slate quarries, or during the large-scale construction of forestry tracks during the 1960s.
Silicon, as silica (another name for silicon dioxide) is also important to certain organisms. In particular diatoms and sponges.
Diatoms are single-celled microscopic algae with a complex cell wall made of silica. They are abundant in all waters, produce oxygen and are food for other aquatic organisms. Diatoms are also frequently used to monitor water quality.
Sponges build their skeletons from a framework of tiny elements called spicules, which are made of silica in most sponge groups. One of the most beautiful examples is the Venus’ Flower Basket glass sponge, which lives anchored to the deep ocean floor near the Philippines. A pair of shrimps lives inside this sponge, breeding inside it and spending their whole lives protected within its delicate glass walls. Thanks to this unusual symbiotic relationship, the dead skeletons of Venus’ Flower Baskets are a popular wedding gift in Japan.
Sponges are the most primitive kind of animal on Earth, and their resistant spicules are found as fossils from as far back as 580 million years ago. Silica is also important in the preservation of other types of fossil. When dead animals or plants are buried, silica from groundwater can fill in the pores and other empty spaces in wood, bone or shells, and/or it can replace the original remains as they decay or dissolve. This is most common in areas where the groundwater has high silica levels, due to volcanic activity or erosion of silica-rich rocks. The organic remains act as a focal point for silica formation, and often the rock surrounding the fossils is made of different minerals. For example, shells that were originally made of calcium carbonate can dissolve and be replaced by silica, whilst being fossilised within limestone (calcium carbonate). Extracting the fossils is a simple process of putting the rock in some acid and waiting for it to dissolve, leaving behind the silicified fossils. The Museum’s fossil collections include many silicified shells of brachiopods, ammonites, bryozoans and other sea creatures.
One of the most spectacular types of fossil preserved in silica is ‘petrified wood’. Silica replaced the original cells of the wood as it decayed and also filled in any gaps, literally ‘turning it to stone’. In some places, including Patagonia and the USA, whole tree trunks replaced by silica are found in so called ‘petrified forests’. Other plant fossils, such as cones, can also be fossilised in this way.
Chert is a rock made of very small crystals of silica. Many major chert deposits formed at the bottom of ancient oceans from ‘siliceous ooze’, which is made of the skeletal remains of millions of tiny organisms including diatoms and radiolarians (single-celled plankton). Chert nodules can also form within other rocks through chemical processes.
Chert found within chalk is known as flint, and was a very important material for making tools throughout Prehistory. Tools are made by knapping, that is striking a prepared flint edge, or striking platform, with a harder stone to detach pieces called flakes or blades. These flakes, blades, and indeed the core from which they are struck can then be modified with secondary working into fine tool forms. Amongst the most skilful are fine arrowheads, including these from a Bronze Age grave at Breach Farm, Vale of Glamorgan, Wales. Flint was generally the material of choice for making sharp cutting tools as it is so fine-grained and fractures conchoidally and cleanly it gives a really sharp cutting edge. Indeed, so much so, that anecdotally eye-surgeons are reported to occasionally use a freshly struck flint blade in the operating theatre!
Because it is very fine-grained and hard, chert can preserve fossils of very small things from far back in our planet’s history. The oldest potential fossils on Earth are found in cherts, and include the possible remains of bacteria from over 3 billion years ago. Younger fossils, from the Rhynie Chert of northern Scotland, provide a glimpse of one of the earliest land communities, 400 million years ago. Simple plants, and animals including primitive spider-like creatures and scorpions, were preserved in fine detail thanks to silica-rich water from volcanic hot springs.
Opal is a hydrated form of silica, meaning that it contains between 3 and 21% water. Unlike standard silica, it does not have a set crystal form, but some of its forms diffract light, creating a beautiful iridescent effect in a variety of different colours. For this reason, opal has been prized for centuries as a gemstone for making pendants, rings and other jewellery. Australia produces a lot of the world’s opal, and is also a source of rare and spectacular opalised fossils. The shells of invertebrates such as belemnites (prehistoric squid-like creatures), and even dinosaur bones, have been replaced by opal, creating very colourful specimens in a world where fossils are usually grey or brown.